-
PDF
- Split View
-
Views
-
Cite
Cite
Yukihide Iwamoto, Kazuhiro Tanaka, The Activity of the Bone and Soft Tissue Tumor Study Group of the Japan Clinical Oncology Group, Japanese Journal of Clinical Oncology, Volume 42, Issue 6, June 2012, Pages 467–470, https://doi.org/10.1093/jjco/hys059
Close - Share Icon Share
Abstract
The Bone and Soft Tissue Tumor Study Group (BSTTSG) of the Japan Clinical Oncology Group (JCOG) was established in 2002. At present, 26 institutions are participating as active members of the BSTTSG of the JCOG. In 2004, the first BSTTSG trial, JCOG 0304, was initiated. JCOG 0304 was a Phase II study of perioperative chemotherapy with doxorubicin and ifosfamide for patients with operable, high-grade, non-round cell soft tissue sarcomas (T2bN0M0) arising in the extremities. The results of JCOG 0304 suggested the efficacy of the perioperative chemotherapy on operable soft tissue sarcoma in the extremities and led to planning of the subsequent clinical trial for the disease. The BSTTSG has currently been conducting a Phase III trial, JCOG 0905, to investigate the superiority of the addition of ifosfamide to the standard chemotherapy with methotrexate, cisplatin and doxorubicin for operable, high-grade osteosarcoma. The BSTTSG is also conducting a JCOG ancillary study, JCOG 0905-A1, to evaluate the gene expression profiles of osteosarcoma treated in the JCOG 0905 study. The BSTTSG will further try to develop new standard therapy for bone and soft tissue tumors.
INTRODUCTION
Bone tissue sarcomas and soft tissue sarcomas (STS) are very rare malignant tumors, arising from the mesenchymal tissues in any sites of the human body. According to the Soft Tissue Tumor Registry reported by the Musculoskeletal Tumor Committee of the Japanese Orthopaedic Association, only 525 and 1086 cases of bone tissue sarcomas and STS were registered in 2009 in Japan, respectively (1,2). Since bone tissue sarcomas and STS exhibit wide varieties of histological subtypes, the incidence of each subtype of tumor in the specific site is extremely rare. Therefore, it is very difficult to develop novel therapy for such rare tumors, and few clinical studies for bone tissue sarcomas and STS were conducted in Japan.
To establish new standard therapy for such rare malignancies, multicenter prospective studies are mandatory. In 2002, the Bone and Soft Tissue Tumor Study Group (BSTTSG) was organized in the Japan Clinical Oncology Group (JCOG). The BSTTSG has conducted two clinical trials and an ancillary study. The first clinical trial of the BSTTSG, JCOG 0304, was initiated in 2004 and closed in 2008. The second trial JCOG 0905 and its ancillary study JCOG 0905-A1 are currently in patient accrual.
JCOG 0304
STS in adults are divided into two groups: round cell sarcomas and non-round cell sarcomas (NRC-STS). The round cell STS such as rhabdomyosarcoma and extra-skeletal Ewing's sarcoma are sensitive to chemotherapy and radiotherapy, and standard regimens of intensive chemotherapy have been established. On the other hand, NRC-STS are relatively chemo-resistant; thus, the standard therapeutic modality for NRC-STS is surgical resection. Since the outcome of surgery alone for the patients with large (>5 cm), deep, high-grade, that is, Stage III (American Joint Committee on Cancer AJCC 6th edition) NRC-STS remains poor, systemic chemotherapy has been required to improve survival of the patients with Stage III NRC-STS (3).
The efficacy of systemic chemotherapy for Stage III NRC-STS is still controversial. Meta-analysis of 14 randomized clinical trials of doxorubicin-based adjuvant chemotherapy for NRC-STS demonstrated that the adjuvant chemotherapy for NRC-STS had significantly prolonged progression-free survival (PFS), whereas overall survival (OS) was not significantly different between surgery alone and adjuvant chemotherapy. Although these old trials did not use ifosfamide, the analysis concluded that the efficacy of adjuvant chemotherapy for NRC-STS was not established (4). On the other hand, an Italian group conducted a randomized controlled trial of adjuvant chemotherapy using epirubicin and ifosfamide versus surgery alone for Stage III NRC-STS in the extremities. The results revealed the significant improvement of survival in the patients with post-operative chemotherapy (5). Furthermore, the update of meta-analysis of 18 randomized trials of adjuvant chemotherapy has been reported (6). The study demonstrated that both PFS and OS were significantly improved by chemotherapy compared with surgery alone. However, a standard chemotherapy regimen for NRC-STS arising in extremities has not been established yet. We therefore carried out the multicenter Phase II study of chemotherapy for patients with Stage III NRC-STS in the extremities, JCOG 0304 (7).
The aim of JCOG 0304 was to evaluate the efficacy and feasibility of pre-operative and post-operative chemotherapy using doxorubicin and ifosfamide on patients with AJCC Stage III NRC-STS in the extremities. The primary endpoint of the study was the 2-year PFS, and secondary endpoints were response rate of the pre-operative chemotherapy, 3-year PFS, OS, adverse events, post-operative complication rate and pathological response rate. Pre-operative chemotherapy consisted of doxorubicin (60 mg/m2) and ifosfamide (10 g/m2) and was repeated for three courses with a 3-week interval. After the tumor resection was completed, two courses of the same regimen as pre-operative chemotherapy were carried out. The first patient was enrolled in July 2004, and patient accrual was finished in September 2008. Although the follow-up period was still relatively short, the survival outcome of the trial for the patients with high-grade STS in the extremities was far better than expected (8). There was no treatment-related death. Grade 3 or 4 hematological toxicities (leukopenia and neutropenia) were observed in most of the patients. Although the toxicities of the regimen were significant, pre-operative chemotherapy followed by post-operative chemotherapy using doxorubicin and ifosfamide was feasible. The BSTTSG is now planning another clinical trial to develop a novel adjuvant chemotherapy regimen for NRC-STS in the extremities.
JCOG 0905
Osteosarcoma is the most frequent primary malignant bone tumor. The prognosis of osteosarcoma has been greatly improved by intensive systemic chemotherapy and surgery. Recent reports have demonstrated favorable outcome of the current therapy for patients with non-metastatic operable osteosarcoma. The key drugs in the standard chemotherapy for osteosarcoma include high-dose methotrexate, cisplatin and doxorubicin (9). Besides these three drugs, it has been reported that ifosfamide is also active for osteosarcoma (10).
Although neoadjuvant chemotherapy failed to demonstrate superiority in survival or limb-sparing rates compared with the adjuvant chemotherapy (11), pre-operative chemotherapy is thought to be the standard regimen since the response to the neoadjuvant regimen is the strong prognostic factor for osteosarcoma patients. The survival rates of the patients with 90% or more tumor necrosis in the surgical specimen (good responder) were significantly higher than those for patients with lesser tumor necrosis (standard responder) (11,12). However, the effects of the addition of other active drugs, such as ifosfamide, in post-operative chemotherapy for standard responders remain controversial.
We have previously observed the improvement in prognosis of the patients assessed as standard responders after pre-operative chemotherapy with methotrexate, cisplatin and doxorubicin by the addition of ifosfamide in post-operative chemotherapy (13). In the Phase II trial, NECO study, 5-year OS of the patients with a good histologic response to pre-operative chemotherapy was 78.7%, whereas the patients assessed as standard responders also exhibited comparable OS of 89.5%. There were no significant differences between OS of the patients in terms of the response to the pre-operative chemotherapy (13). The results suggested the efficacy of the addition of ifosfamide to three-drug standard chemotherapy. To investigate the impact of ifosfamide on the patients with osteosarcoma, the BSTTSG is currently conducting the randomized trial JCOG 0905.
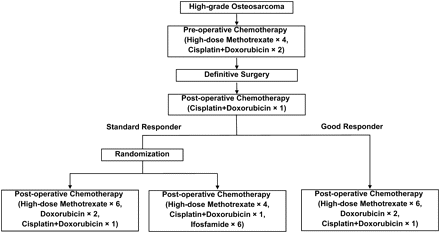
In the JCOG 0905 study, the patients with high-grade osteosarcoma in the extremities are initially treated with high-dose methotrexate, cisplatin and doxorubicin. After the completion of tumor resection, the patients assessed as good responders are treated with the same three-drug regimen as pre-operative chemotherapy. The patients assessed as standard responders are randomly assigned to receive or not receive the addition of ifosfamide. The study design of JCOG 0905 is shown in Fig. 1. JCOG 0905 was activated in 2010, and the expected period for accrual completion is 6 years.
JCOG 0905-A1
The efficacy of chemotherapy for osteosarcoma is well established; however, the prediction of the response or survival benefit in each patient at diagnosis is difficult. Although pathologic tumor necrosis would be a powerful predictor of survival, the prognostic marker could only be determined after induction therapy has already been given. Therefore, this marker is not a true prognostic indicator and ineffective in altering the outcome of chemo-resistant tumors. To date, no true prognostic markers have been identified yet.
Recently, several studies were conducted to determine gene expression profiles predictive of poor prognosis and possible therapeutic targets (14–16). However, there were no genes commonly identified among these studies. Thus, the BSTTSG is conducting the gene expression profile analysis to identify true prognostic markers using osteosarcoma samples from the patients participating in JCOG 0905. Since the materials are prospectively collected from the patients treated with the same protocol and the clinical data are precise and reliable, the ancillary study will provide important information regarding true prognostic factors of osteosarcoma.
FUTURE DIRECTION
The BSTTSG is now planning to develop the novel perioperative chemotherapy regimen using docetaxel and gemcitabine for high-grade NRC-STS. Not only for sarcoma, the BSTTSG will also investigate effective therapies for metastatic bone tumors and is planning to carry out a study to develop the standard surgery for cancer metastases to long bones. The BSTTSG will continuously conduct clinical trials to establish more effective treatments for patients suffering from bone and soft tissue tumors.
PARTICIPATING INSTITUTIONS (AS OF JANUARY 2012, FROM NORTH TO SOUTH)
Sapporo Medical University, Hokkaido Cancer Center, Tohoku University, Chiba Cancer Center, National Cancer Center Hospital, Nihon University, Kyorin University, Keio University, Cancer Institute Hospital, Teikyo University, Yokohama City University, Kanagawa Cancer Center, Niigata Cancer Center Hospital, Gifu University, Shizuoka Cancer Center, Aichi Cancer Center, Nagoya University, Mie University, Kyoto University, Osaka University, Osaka Medical Center, Okayama University, Hiroshima University, Kyushu University, National Kyushu Cancer Center and Kurume University.
Funding
This work was supported in part by National Cancer Center Research and Development Fund, the Grants-in-Aid for Cancer Research, and Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare, Japan.
Conflict of interest statement
None declared.